28+ calculate van't hoff factor
Web Vant Hoff factor i. A is the degree of dissociation.

Science Magazin Pdf Juno Spacecraft Open Access
To obtain the integrated equation it is.
. Web Calculate the vant Hoff factor i for MX2 at this concentrationA 121 m aqueous solution of an ionic compound with the formula MX2 has a boiling point of 10155 C. Determine the freezing point of water and the freezing point depression of the two salt. Web The Vant Hoff factor i is a determining factor on how solutes affect the collaborative characteristics of solutions.
Web Calculate the vant hoff factor for a 0050 m aqueous solution of m g c l 2 that has a measured freezing point of 025c. Of moles of solute after dissociationassociation divided by total no. This change in enthalpy can be positive or negative.
Web You can only determine these experimentally or find a table of vant Hoff factors for a give concentration. I an 1 a where i is the Vant Hoff Factor. Web Description The Van t Hoff equation in chemical thermodynamics relates the change in the equilibrium constant Keq of a chemical equilibrium to the change in temperature T.
Here we will use ideal van t Hoff factors. Web The actual van t Hoff factor is thus less than the ideal one. 20 g 1mole5844 g.
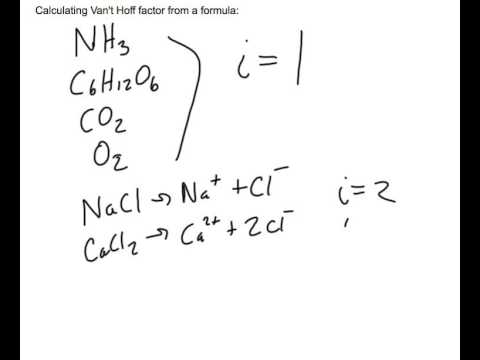
The vant Hoff factor i is the number of particles formed in a solution from one formula unit of solute. Web The following formula can be used to calculate a Vant Hoff Factor. I moles of particles in solution moles of formula units dissolved.
Web One of the most common formulas used to calculate the value of the Vant Hoff factor is i moles of particles in solutionmoles solute dissolved. Of moles of solute before. I actual number of particles in solution after dissociation number of formula units initially dissolved in.
Convert 20 g of NaCl to moles. N is the number. Revised equations to calculate the effect of ionization are.
Web To be quantitative we introduce the vant hoff factor i. Web The Vant Hoff factor can be defined as the ratio of the concentration of particles formed when a substance is dissolved to the concentration of the substance by mass. Web Be sure to title your plot clearly label each data series and include axes labels.
Web The Van t Hoff plot can be used to quickly determine the enthalpy of a chemical reaction both qualitatively and quantitatively. Vant Hoff Factor for the. Notice that i is a property of the solute.
I δTb Kb x molality Where. Web The van t Hoff factor is the ratio between the actual concentration of particles produced when the substance is dissolved and the concentrationof a substance as calculated from. Web The vant Hoff factor i is defined as the ratio of solute particles in solution to the number of formula units dissolved.
Web Calculating the Vant Hoffs Factor when the Boiling Point elevation Ebullioscopic Constant and Molality is Given.
Ebullioscopy Determination Of The Van T Hoff Factor And Dissociation Degree Of A Dissociated Solute

Pdf Low Ph Induced Conformational Change And Dimerization Of Sortilin Triggers Endocytosed Ligand Release
Van T Hoff Factor Formula Physics Wallah

Van T Hoff Factor Calculator Calculator Academy

Thermodynamics Of Cement Hydration Pdf Cement Scanning Electron Microscope
Finding The Van T Hoff Factor Youtube

How To Calculate Van T Hoff Factor I Youtube

Van T Hoff Factor I How To Calculate Van T Hoff Factor I Entrancei

Math Physics Chemistry Questions Discussion Lists Dated 2019 05 18

Chem 201 Calculating Actual Van T Hoff Factor Youtube

Van T Hoff Factor I How To Calculate Van T Hoff Factor I Entrancei
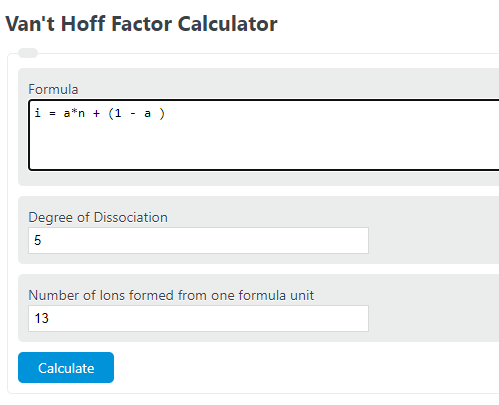
Van T Hoff Factor Calculator Calculator Academy

Finding The Van T Hoff Factor Youtube

Chapter Wise Dpp Sheets For Che Disha Experts 2 Pdf
How To Calculate The Van T Hoff Factor Of A Solute Quora

The Van T Hoff Factor Definition And How To Calculate It

Pdf Low Ph Induced Conformational Change And Dimerization Of Sortilin Triggers Endocytosed Ligand Release